We have a new paper now published at Communications Biology. It can be read here.
In this paper, we investigated the question of whether the number of action potentials emitted by a superior colliculus (SC) neuron at the time of saccadic eye movement triggering is related to the eyeball rotation speed of the generated saccade. This idea has been proposed by numerous accepted models of saccade control by the SC. We tackled this question using two complementary approaches.
First, we found that upward saccades have weaker saccade-related motor bursts in the SC than downward saccades (i.e. neurons driving upward saccades emit fewer action potentials in their saccade-related motor bursts than neurons driving downward saccades). This is consistent with our earlier work.
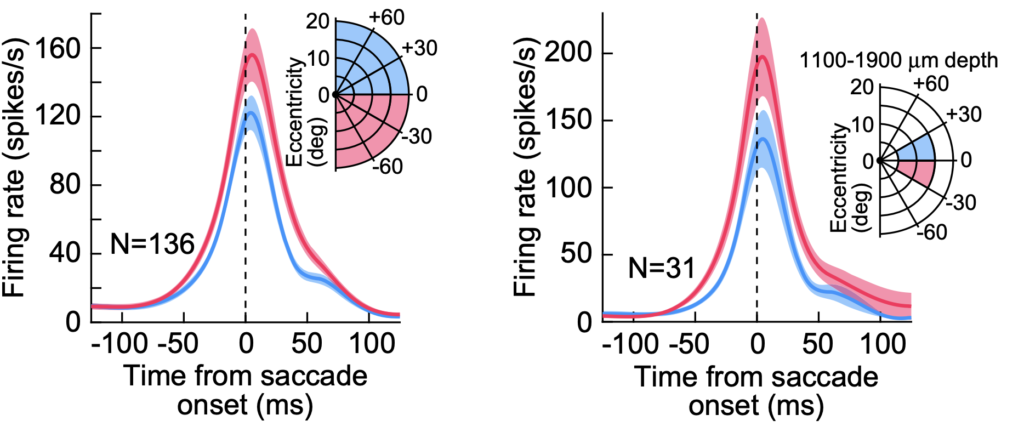
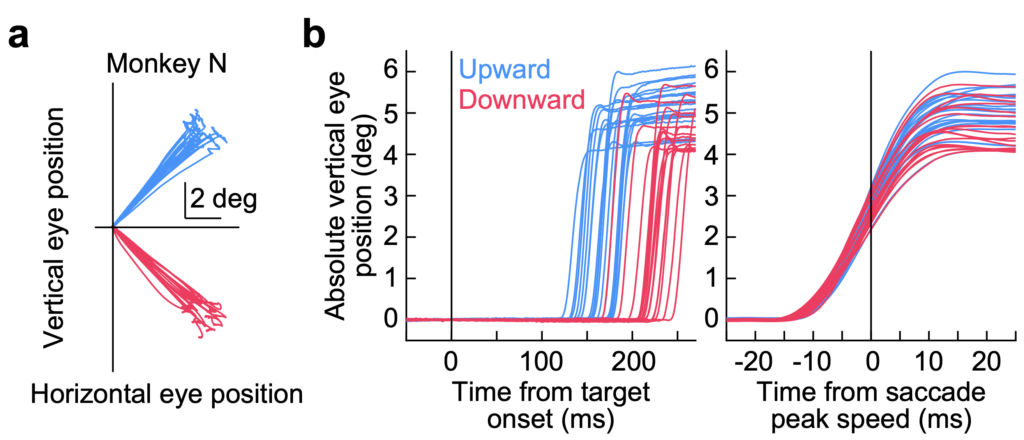
If the number of action potentials in the motor bursts dictates movement kinematics (i.e. saccade speed), then downward saccades should be systematically faster in peak speed than upward saccades. However, this is not what we found. Even though the saccade reaction times are slower for downward saccades (through mechanisms not related to the motor bursts; see here), the kinematics of downward saccades were similar to those of upward saccades.
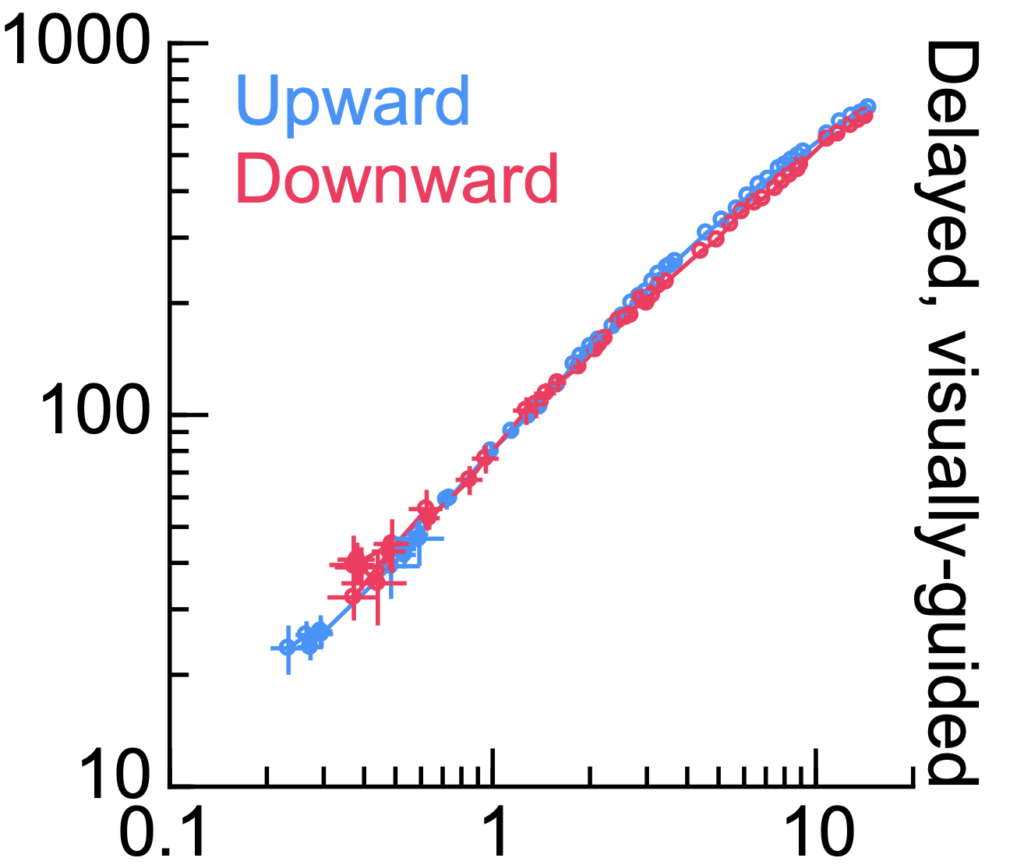
We also used a second approach to test the hypothesis. We used vector-matched saccades to a visible spot or to a blank. The latter saccades have slower speeds than the former saccades, even if they go to exactly the same location in space. This is a known property of saccades to a blank. If SC motor bursts correlate in individual neurons with saccade speed, then SC motor bursts should be systematically weaker for saccades to a blank than for saccades to a visible spot. This is not what we found. We found a diversity of effects on SC motor bursts, with some neurons actually emitting more action potentials for the slower saccades to a blank.
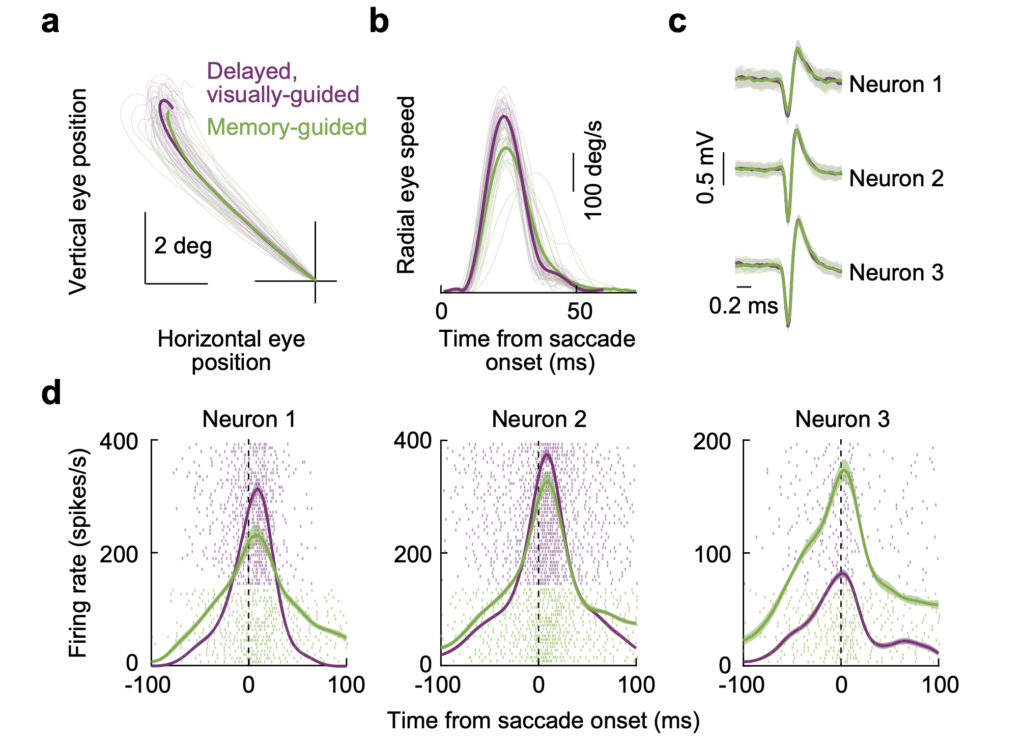
Thus, there is a strong dissociation between SC motor burst strength and saccade kinematics. We found other related evidence to this idea in our earlier work. For example, we recently altered saccade metrics and kinematics, but the SC motor bursts were not affected.
Importantly, this current study provides important foundational work for our latest discovery, because that latest discovery also argues that SC motor bursts are dissociated from eye movement kinematics.
