We have a new paper just published in iScience!
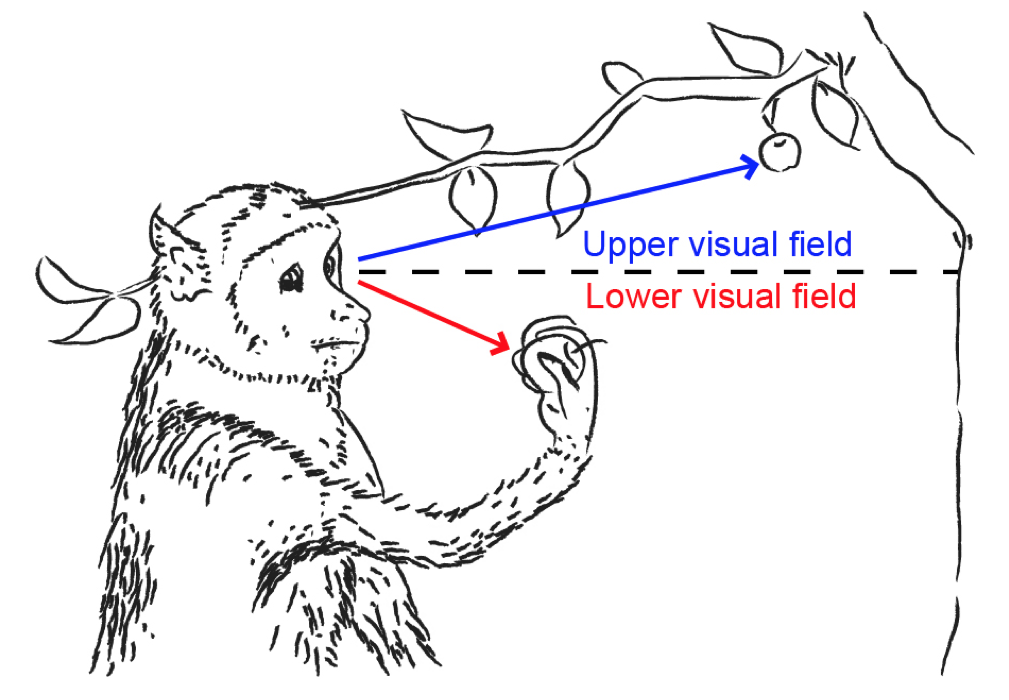
In 2016, we published an Article in Current Biology describing a large and surprising asymmetry in how the primate superior colliculus (SC) represents the upper and lower retinotopic visual fields. Specifically, we discovered, back then, that SC visual responses are much stronger in the SC’s upper visual field representation than in its lower visual field representation, and we linked this to the importance of eye movements in facilitating the “remote sensing” of extra-personal, “far”, space (largely occupying the upper visual field relative to our line of sight).
More recently, in an Article in PNAS, we made another equally surprising discovery about the primate SC, this time related to its saccade-related motor bursts. We found that these saccade-related motor bursts exhibit paradoxical visual feature tuning properties in them, such that the same eye saccade (both in metrics and kinematics) to two different visual images would be associated with two distinct motor bursts by the very same SC neuron.
While this newer discovery has many different implications that we are actively investigating, it has prompted us to, specifically, revisit the question of visual field asymmetries in the SC, but now from a very different perspective. We wondered whether the sensory preference for the upper visual field in the SC would persist even at the time of saccade generation. We made a most remarkable discovery, which we now just published!
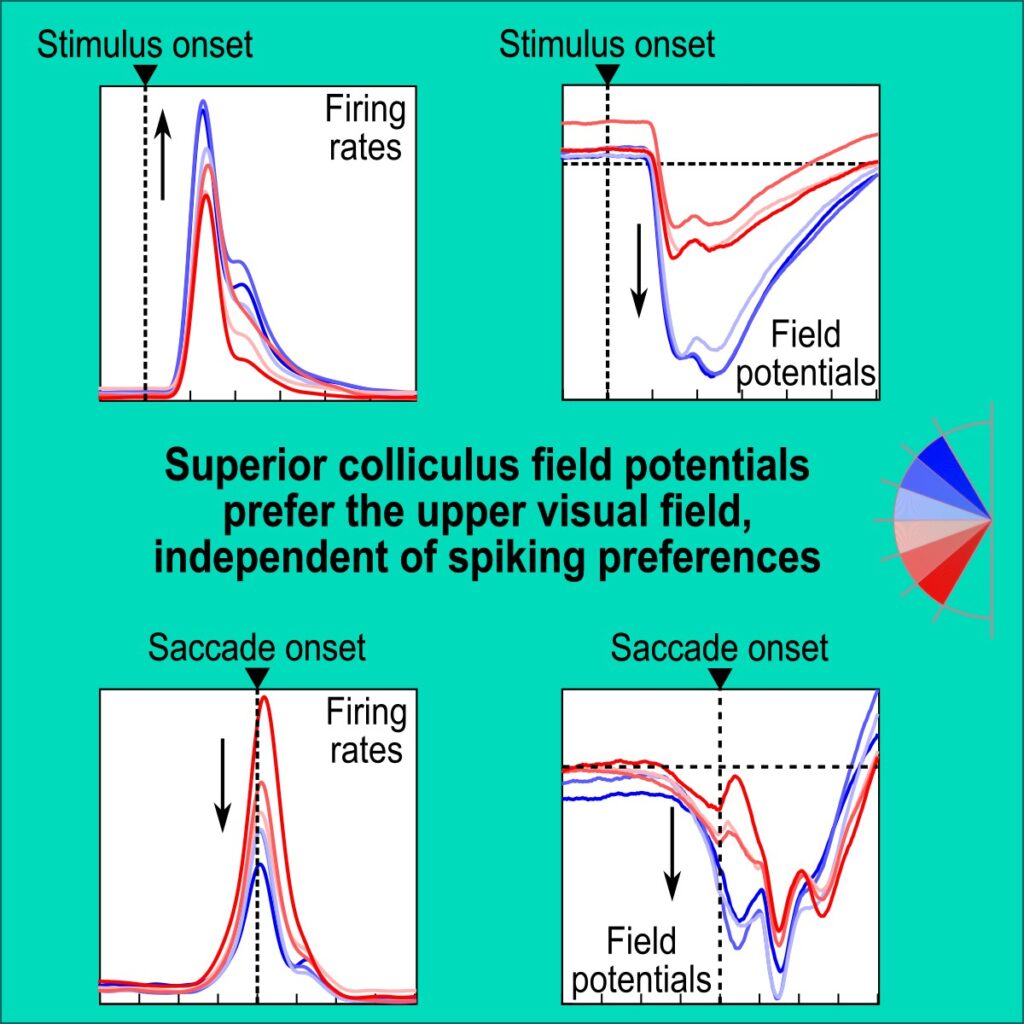
We analyzed peri-saccadic local field potentials (LFP’s) in the SC, and we did so from SC sites representing either the upper or the lower retinotopic visual field. It was known for a few years now that SC LFP’s do exhibit robust peri-saccadic modulations that may appear qualitatively similar to the spiking activity in the concomitant motor bursts. For example, there is an LFP negativity that is time-locked to saccade onset, just like there is a firing rate burst also time-locked to the very same eye movement event. However, when we checked the properties of this peri-saccadic LFP negativity as a function of the SC’s topographic visual field map, we were surprised to uncover a major dissociation from the motor bursts: saccade-aligned LFP negativity was much stronger for upper visual field SC sites than for lower visual field sites, which was the exact opposite of how the motor bursts behaved. Thus, the SC network maintains a very strong sensory preference for the retinotopic upper visual field exactly at the time of the saccade motor bursts.
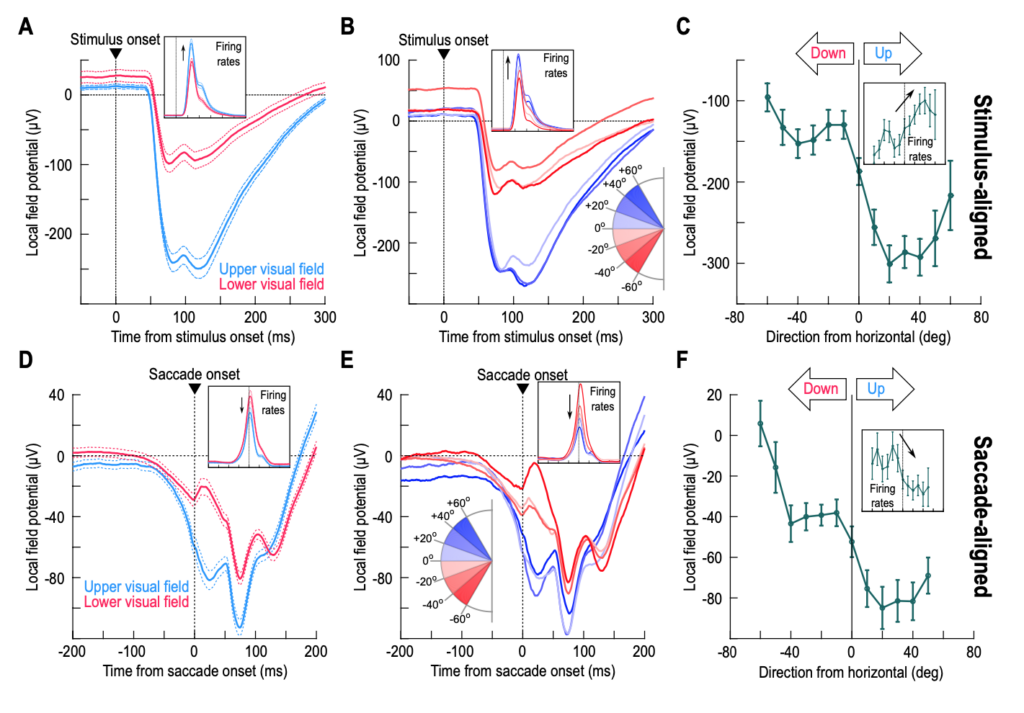
This discovery then allowed us to test saccades towards a blank instead of towards a visible target. We discovered the same preference in peri-saccadic LFP’s for the upper visual field, suggesting that it was the SC’s topographic location of the neurons that mattered the most for this novel phenomenon. We also found that the memory representation itself in the SC was dependent on whether the neurons represented the upper or lower visual field.
Most importantly, based on this, we predicted (and validated) that motor bursts would still be weaker in the upper visual field with memory-guided saccades towards a blank region in the environment, instead of towards a visible target.
The dissociation between LFP’s and spiking activity that we reported in our paper is the largest and most compelling that we have seen in the oculomotor literature so far. For example, there have been reports of how timing relationships between LFP negativity and spiking bursts in the SC and frontal eye fields are jittered between the visual response and saccade-related epochs in active oculomotor behavior. However, we saw a complete reversal in the motor epoch: SC motor bursts are weak for upper visual field saccades, but SC peri-saccadic LFP negativity is very strong; this reversal is not present in the visual response epoch. Thus, the sensory preference for the upper visual field in the SC’s LFP signals is omnipresent in both visual and saccade-related motor epochs.
